DAS in Translational Medicine and Biomedical Entrepreneurship
School for Translational Medicine and Biomedical Entrepreneurship (sitem-insel School)
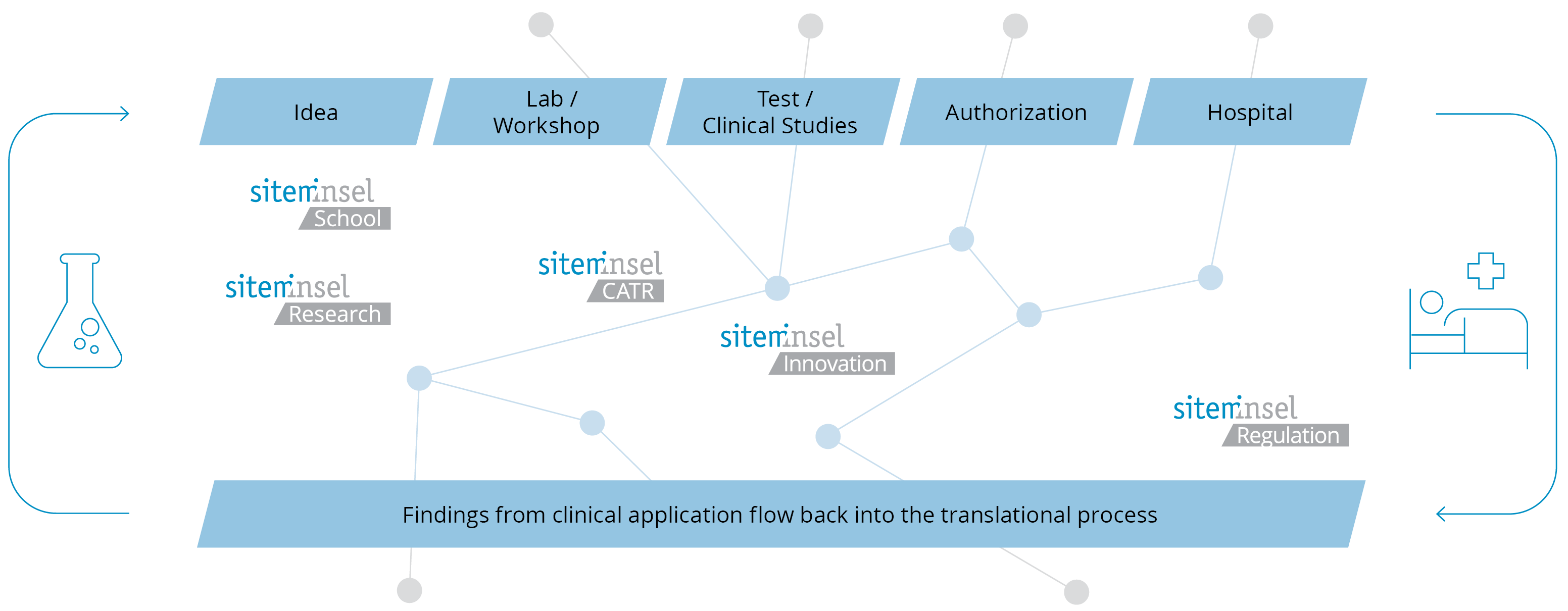
Translational medicine is a new, process-oriented discipline that aims to translate new findings and products emerging from private-sector development and basic research into clinical applications. It seeks to professionalize the essential interaction between basic science researchers, clinicians, regulatory bodies and investors.
This study program is designed to train professionals in scientific and medical knowledge, and simultaneously in entrepreneurship skills to enable them to successfully coordinate the development and commercialization of biomedical products.
Aspiring to change the future of healthcare?
Translational medicine is a new, process-oriented discipline that aims to translate new findings and products emerging from private-sector development and basic research into clinical applications. It seeks to professionalize the essential interaction between basic science researchers, clinicians, regulatory bodies and investors.
This study program is designed to train professionals in scientific and medical knowledge, and simultaneously in entrepreneurship skills to enable them to successfully coordinate the development and commercialization of biomedical products.
| Degree | Diploma of Advanced Studies in Translational Medicine and Biomedical Entrepreneurship, Universität Bern (DAS TMBE Unibe) |
|---|---|
| Start | 2026 |
| Length | 3 Semester |
| Scope | 30 ECTS |
| Cycle | Annual |
| Flexible entry possible | Yes |
| Single module visitable | Yes |
| Place | sitem-insel, Freiburgstrasse 3, 3010 Bern, Switzerland |
| Language | English |
| Admission | Min. Bachelor's or higher degree in life science, medicine, engineering science or equivalent and at least 2 years of experience in research and/or development. “Sur dossier” admissions possible. |
| Registration until | 2026/07/15 |
| Cost | CHF 23'100 |
| Organising institutions | School for Translational Medicine and Biomedical Entrepreneurship (sitem-insel School) |
About the program
Translational medicine is a new, process-oriented discipline that aims to translate new findings and products resulting from industrial development and basic research into clinical applications. It seeks to professionalize the essential interaction between research, clinics, regulatory bodies, investors and industry. This two-year part-time program study program is designed to train professionals in scientific-technical knowledge, and simultaneously in entrepreneurial skills to enable them to successfully coordinate the development and commercialization of their healthcare innovation.
What to expect from this program?
- Take your innovative idea and boost the success of your start up
- Become proficient in navigating the landscape and interdisciplinary interaction along the translational pathway
- Increase your career prospects
- Obtain an internationally recognised degree from a prestigious Swiss university
- Gain expert insights from academia and industry
- Connect and exchange with peers, lecturers and experts from industry
- Acquire extensive theoretical knowledge and practical skills in topics relevant to your project
- Profit from small class sizes, individual coachings and flexible study formats
Who is the program for?
The program addresses researchers from various fields of medicine, pharmacy, natural sciences or engineering, who are eager to translate their research-based innovation projects into a succesful startup. It is designed to foster and develop startup ideas towards a marketable product by directly applying the knowledge acquired during the program to the individual project. The program also addresses professionals from industry, hospitals and academia that are active in the medical sector seeking to gain comprehensive knowledge and practical skills in translational medicine and biomedical entrepreneurship.
After successful participation, the University of Bern awards the title Diploma of Advanced Studies with a diploma supplement disclosing the contents and achievements of the program.
Program structure
The DAS TMBE is a two-year part-time program, consisting of at least 4 modules and a DAS thesis. The theoretical knowledge acquired during the modules is directly and specifically applied to the individual projects.
The program scope corresponds to min. 30 ECTS, with one ECTS being estimated a study work performance of about 25-30 hours, including in-person events, preparatory tasks and all other course activities. The program is conceptualized as an extra-occupational program that can be reconciled with your professional work. The modules are taught in a blended learning environment and utilize e-learning, peer learning and interactive discussions with experts, on site lectures, workshops, and case studies. Small class sizes allow for large flexibility while at the same time permitting to profit from the lecturers’ expertise.
The continuing education program in Translational Medicine and Biomedical Entrepreneurship has a modular structure, allowing individual learning approaches and fast tracks (see MAS TMBE, DAS TM, CAS TM, CAS BE). It is also possible to book single modules in case free places are available.
After successful participation, the University of Bern awards the title Diploma of Advanced Studies in Translational Medicine and Biomedical Entrepreneurship.
Modules
Participants of the DAS in Translational Medicine and Biomedical Entrepreneurship choose three out of the five modules with technical-scientific focus (i.e. M1-M5) and complete the full module with strategic-entrepreneurial focus (i.e. M6):
TMBE Scholarship Program
Organising institution and faculty
Swiss Institute for Translational and Entrepreneurial Medicine (sitem-insel)
sitem-insel is the Swiss Institute for Translational and Entrepreneurial Medicine. It is located on the Insel Campus Bern and benefits from its proximity to Switzerland's largest university hospital (Inselspital) as well as the University of Bern. At sitem-insel, a wide variety of units from clinics, industry, research, and education are networked under one roof, driving innovation for the benefit of patients. Our mission is to establish, operate and develop a National Center of Excellence for Translational Medicine that professionalizes translational research for the benefit of patients, society, and science. An onsite cutting-edge 20,000 m2 facility is the sitem-insel catalyst for a multidisciplinary collaborative approach to unlocking ‘bench to bedside’ innovation.
Bringing Innovation to the Patient – by Connecting People.
How to get a research idea from bench to bedside?
Together with you, we are committed to bridging the gap between clinical practice, research, entrepreneurship and regulation.
Medical Faculty of the University of Bern
Our education programs in translational medicine, biomedical entrepreneurship, regulatory affairs and artificial intelligence are offered under the umbrella of the Medical Faculty of the University of Bern.
The Medical Faculty is known for an excellent curriculum in human and dental medicine, and also offers an attractive and high-quality range of training and continuing education in the field of health and medicine. With several institutes and clinics in various disciplines, it has excellent, internationally recognized research performance. It also conducts cutting-edge research in artificial intelligence for medicine and provides access to data science for all research groups.
Experts from industry and academia
Our experts from academia, clinic, industry and regulatory authorities, carry the teaching responsibility and support us in educating healthcare and regulatory specialists, bridging knowledge gaps, and advancing professional careers. They combine deep theoretical knowledge from their innovative research, with the latest practical insights from their extensive work with industry.
Admission, fees and installments
Admission requirements
Applicants must hold at least a Bachelor's degree from a university or university of applied sciences in the fields of natural sciences and engineering, medicine, pharmacy or law. Professional experience is not mandatory. If the applicant has no prior academic degree or professional experience, the study commission may define further conditions for the applicant to successfully complete the course.
Admission process
Please submit your complete application including all required attachments via the registration form. Extensions of application deadlines may be granted by the Directorate.
To complete the application, you will need the following documents:
- Copy of your resume/curriculum vitae (CV)
- Recent passport photo (see guideline) and a copy of the passport/ID
- Signed copy of Confidentiality Declaration (see documents below)
- Copy of highest relevant academic degree
Once we have received your application, we will gladly evaluate your documents and get back to you within a few working days. Upon successful registration, you will get a Campus Account from the University of Bern, which allows access to the student area of the University websites, the University´s WLAN network (eduroam), the use of library databases and of e-journals. MAS students will also receive a UNICARD and have access to sports, childcare and counselling facilities offered by the University of Bern.
Deadlines
Application for scholarship: May 31
Application for fall term: July 15
Application for spring term: December 15
Standalone modules can be admitted anytime, provided places are still available.
The Directorate may grant exceptions on the above deadlines.
Tuition fees & installments
Master of Advanced Studies (MAS): CHF 31'500.–
Diploma of Advanced Studies (DAS): CHF 23'100.–
Certificate of Advanced Studies (CAS): CHF 12'600.–
The full study fee can be paid in full at once or in installments per semester.
There is no entitlement to a refund or waiver of the course fees if parts of the course are not attended. Costs for travel, accommodation and catering are not included. Insurance is in the sole responsibility of participants (accident, travel, cancellation, etc.).
Withdrawal of the registration before the registration deadline is possible without cost consequences. In case of withdrawal after the registration deadline, the full course fee must be paid.

Gordana Veljanoska
«Working on disrupting technologies at a MedTech company made me understand the challenges that come when facing the regulatory environment and the clinical application of a device. This is exactly where the sitem-insel School plays a crucial role, enabling me to directly apply knowledge to practical case scenarios with continuous support from industry professionals. This is what I enjoy most about the MAS, as well as the flexibility and the project-oriented case studies.»
Regulatory & Clinical Affairs Specialist, CASCINATION
Giacomo Valle
«The TMBE program has helped MYLEG in the transition from a university-based project to entrepreneurial reality. With the support of a network of experts and advisors both in science and business, we have achieved important milestones which are fundamental to enter the medical device market. We have developed a unique and competitive product that improves the lives of people with disabilities using robotics and personalized neurostimulation.»
Founder of MYLEG


Florence Von Gunten
«The TMBE program helped me greatly with the development of YLAH - blended psychotherapy, our University of Bern spin-off. We found the module content to be highly relevant for our digital therapeutic product. The applied exercises and the personal support by the course instructors have helped us to make substantial progress. We have gained a solid theoretical basis and a network on which we can continue to build our MedTech startup.»
Co-Founder of YLAH
Dr. c. Raja Prince-Eladnani
«I was able to directly apply what I learned through each module and expand my skills in regulatory affairs, project management, business development and entrepreneurship.»
Clinic of Hematology & Central Hematology Laboratory, DBMR, University of Bern and Inselspital Bern

Associate Courses
CAS in Artificial Intelligence in Medical Imaging
| Degree | CAS |
|---|---|
| Start | 2026 |
| Language | Englisch |
| Cost | CHF 10'080.- |
The Certificate of Advanced Studies in Artificial Intelligence in Medical Imaging (CAS AIMI) offers a hands-on, application-focused introduction to Artificial Intelligence (AI) in the field of medical imaging. Designed specifically for professionals without relevant technical background, it provides the foundational understanding and practical skills needed to critically assess, use, and contribute to AI solutions in clinical and research environments. Through real-world case studies and guided exercises, participants will learn how AI is transforming diagnostic imaging and healthcare delivery.
CAS in Translational Medicine
| Degree | CAS |
|---|---|
| Start | 2026 |
| Language | Englisch |
| Cost | CHF 12'600 |
The program includes all relevant aspects of the translational process, including basic scientific, clinical, and technical knowledge. It covers different points relevant for the development and commercialization of medicinal products and medical devices.
CAS Biomedical Entrepreneurship
| Degree | CAS |
|---|---|
| Start | 2027 |
| Language | Englisch |
| Cost | CHF 12'600 |
This program equips participants with the necessary entrepreneurial know-how and skills to bring a research based innovation to the market.
MAS in Translational Medicine and Biomedical Entrepreneurship
| Degree | MAS |
|---|---|
| Start | 2026 |
| Language | Englisch |
| Cost | CHF 31'500 |
The program includes all relevant aspects of the translational process – entrepreneurial know-how in addition to all basic scientific, clinical, and technical knowledge. It covers different points relevant for the development and commercialization of medicinal products and medical devices.